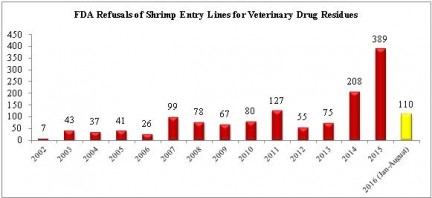
Over the holiday weekend, the U.S. Food and Drug Administration (FDA) released information regarding imports refused entry into the United States for the month of August. In total, the agency reported refusing 148 seafood entry lines last month. Of these, 31 (20.9%) were of shrimp entry lines refused for reasons related to banned antibiotics.
The 31 refusals in the month were the highest for any month in 2016 so far this year. With four months still to go, the FDA has reported refusing 110 total entry lines of shrimp for veterinary drug residues. This is the fourth highest number of such refusals for any year over the last fifteen years.
The elevated level of refusals may reflect improved targeting techniques developed by the agency to address problematic shipments. Further, the refusals reported for August provide strong evidence that the FDA has improved coordination among the agency’s regional district offices to discourage port shopping.
In August, the FDA reported refusing shrimp entry lines originating from three different companies from three different countries (India, Vietnam, and Malaysia) for banned antibiotics. Notably, refusals were reported at multiple ports for each company.
- Jagadeesh Marine Exports (India), a company not currently listed on Import Alert 16-129 and that recently had its November 16, 2015 listing on Import Alert 16-124 for chloramphenicol removed, had eleven entry lines refused for shrimp contaminated with veterinary drug residues in the New York District; six entry lines refused for shrimp contaminated with veterinary drug residues in the Atlanta District; and three entry lines refused for shrimp contaminated with veterinary drug residues in the Southwest District;
- Tan Phong Phu Seafood Company (Vietnam), a company not currently listed on Import Alerts 16-124 or 16-129, but became the first Vietnamese company listed on Import Alert 16-127 for chloramphenicol in its shrimp on March 30, 2016, had six entry lines refused for shrimp contaminated with chloramphenicol and salmonella in the Los Angeles District; two entry lines refused for shrimp contaminated with veterinary drug residues and salmonella in the Atlanta District; and one entry line refused for shrimp described as “poisonous” that was contaminated with chloramphenicol and salmonella in the Chicago District; and
- Hai Soon Leong Sdn. Bhd. (Malaysia), a company located in Peninsular Malaysia that is not currently exempted from Import Alert 16-136, had one entry line refused for shrimp contaminated with nitrofurans and veterinary drug residues in the New York District; and one entry line refused for shrimp contaminated with veterinary drug residues in the Los Angeles District.
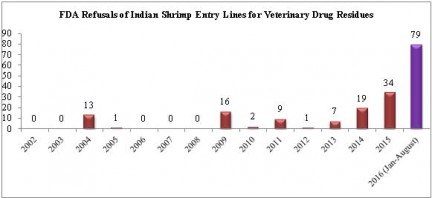
Once again, refusals of entry lines of shrimp from India dominated shrimp refusals for reasons related to veterinary drug residues in August. The number of shrimp entry lines from India refused for these reasons in 2016 is now more than double any prior year for which information is available:
One single company, Jagadeesh Marine Exports,accounts for 49 of those 79 refusals.