Today, the U.S. Food and Drug Administration (FDA) published information regarding entry line refusals for the month of October. The agency reported refusing a total of 112 seafood entry lines last month. Of these, 17 (15.2%) were of shrimp entry lines refused for reasons related to banned antibiotics.
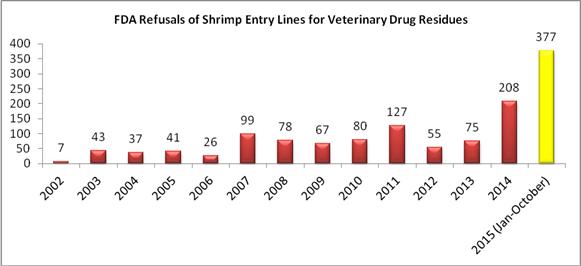
Through the first nine months of 2015, the FDA has now refused a record total of 377 entry lines of shrimp products for reasons related to banned antibiotics.
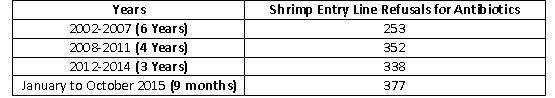
Cumulatively, the FDA’s refusals of shrimp entry line for reasons related to banned antibiotics in 2015 substantially exceeds the agency’s actions over the prior thirteen years. As the table below indicates, the 377 shrimp entry line refusals in just the first nine months of this year exceeds the total number of such refusals in the six years between 2002 and 2007, the four years between 2008 and 2011, and the three years between 2012 and 2014:
The seventeen entry line refusals in October involved shrimp shipped from six different companies located in India, Malaysia, and China. The refusals were reported from five different FDA District Offices spread across the country:
- Kay Kay Exports (India), a company listed on Import Alert 16-129 for nitrofurans on September 17, 2015 but not currently listed on Import Alert 16-124, had
two entry lines refused for shrimp contaminated with both nitrofurans and veterinary drug residues in the New York District;
- RDR Exports (India), a company listed on Import Alert 16-129 for nitrofurans on October 9, 2015 but not currently listed on Import Alert 16-124, had
one entry line refused for shrimp contaminated with nitrofurans in the Los Angeles District;
- Ocean Vision Sdn. Bhd. (Malaysia), a company listed on Import Alert 16-129 for nitrofurans on June 9, 2015 but not currently listed on Import Alert 16-124, had
three entry lines refused for shrimp contaminated with both nitrofurans and veterinary drug residues in the San Francisco District and two entry lines refused for shrimp contaminated with both nitrofurans and veterinary drug residues in the Los Angeles District;
- Omega Frozen Seafood Sdn. Bhd. (Malaysia), a company listed on Import Alert 16-129 for nitrofurans on August 11, 2015 but not currently listed on Import Alert 16-124, had
two entry lines refused for shrimp contaminated with both nitrofurans and veterinary drug residues in the New York District;
- Ruian Huasheng Aquatic Products Factory (China), a company not currently exempted from Import Alert 16-131 (covering shipments of certain types of aquacultured seafood, including shrimp, from China), had
five entry lines refused for shrimp contaminated with veterinary drug residues in the Baltimore District; and
- Zhousahn Yueyang Food Co., Ltd. (China), a company not currently exempted from Import Alert 16-131 (covering shipments of certain types of aquacultured seafood, including shrimp, from China), had
two entry lines refused for shrimp contaminated with veterinary drug residues in the San Juan (Puerto Rico) District.
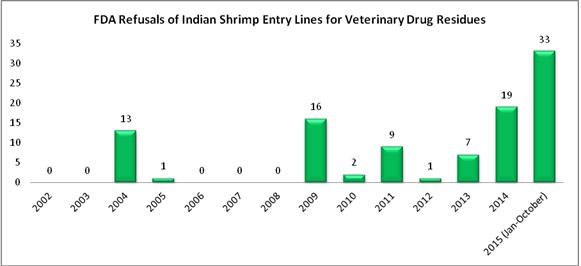
Beyond the record number of entry line refusals of shrimp from all countries for reasons related to antibiotics, as shown in the chart below, through the first nine months of 2015, the FDA has refused more entry lines of shrimp for antibiotics from India (33) than the agency refused in the previous three years combined (27).