The U.S. Food and Drug Administration (FDA) has published detailed data regarding 117 total seafood entry line refusals in September (44) and October (73), of which just one (0.9%) was of shrimp for reasons related to banned antibiotics.
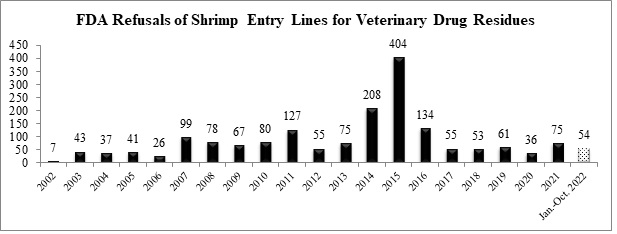
Through October of this year, the FDA has refused a total of 54 entry lines of shrimp for reasons related to banned antibiotics. With just two months left in the calendar year, it appears likely that the total number of entry line refusals of shrimp for veterinary drug residues will be significantly below last year’s total of 75.
The one shrimp entry line refused for antibiotic residues in October was for a shipment from a company located in Mexico:
- Congeladora Navamar (Mexico), a company that is not currently listed on Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”), Import Alert 16-127 (“Detention Without Physical Examination of Crustaceans Due to Chloramphenicol”), or Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”), had one entry line refused for shrimp contaminated with nitrofurans and veterinary drug residues by the Division of Southwest Imports on October 19, 2022.
Although just one entry line of shrimp was refused by the FDA over the last two months for veterinary drug residues, another five entry lines of shrimp were refused for the presence of salmonella, while seven entry lines of shrimp were refused for being filthy.
