Earlier today, the U.S. Food and Drug Administration (FDA) published detailed data regarding 49 total seafood entry line refusals in February, of which 16 (32.7%) were of shrimp for reasons related to banned antibiotics.
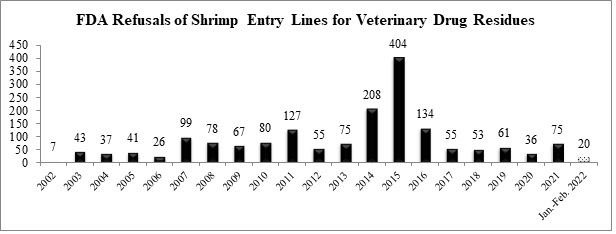
Over the first two months, the FDA has refused a total of 20 entry lines of shrimp for reasons related to banned antibiotics, down from 27 entry lines over the same time period last year.
The sixteen shrimp entry lines refused in February were for shipments from India, Thailand, and Vietnam:
- Aquatica Frozen Foods Global Pvt. (India), a company that is currently listed on Import Alert 16-127 (“Detention Without Physical Examination of Crustaceans Due to Chloramphenicol”) as of March 3, 2022, had four entry lines refused for shrimp contaminated with chloramphenicol by the Division of Northeast Imports on February 24, 2022;
- Good Luck Product Co., Ltd. (Thailand), a company that is currently listed on Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”) as of January 25, 2022 for Gentian Violet in its shipments of shrimp, had five entry lines refused for shrimp contaminated with veterinary drug residues by the Division of West Coast Imports on February 9, 2022 and another six entry lines refused for shrimp contaminated with veterinary drug residues on February 18, 2022; and
- Cadovimex Seafood Import-Export Processing (Vietnam), a company that is currently listed on both Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”) as of June 9, 2017 for enrofloxacin, ciprofloxacin, and sulfadiazine for its shipments of tilapia and Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) as of July 14, 2021 for its shipments of freshwater shrimp (Macrobrachium rosenbergii), had one entry line refused for shrimp contaminated with nitrofurans and veterinary drug residues by the Division of West Coast Imports on February 1, 2022.
The FDA’s inclusion of Aquatica Frozen Foods in Import Alert 16-127 yesterday (March 3, 2022) marked the first time that a company had been added to the Import Alert covering chloramphenicol for its shipment of shrimp since August 2018. Further, the agency’s listing of Good Luck Product Co., Ltd. in Import Alert 16-124 in January marked the first time that a company had been added to the Import Alert covering unapproved drugs for the presence of Gentian Violet in its shrimp since September 2009.
Moreover, with a total of eleven (11) refusals of entry lines of shrimp from Thailand in February, the FDA announced nearly as many refusals of Thai shrimp entry lines for reasons related to veterinary drugs as it reported over the previous seventeen (17) years. Specifically, between 2004 and 2020, the FDA reported refusing a total of just twelve (12) entry lines of shrimp from Thailand for reasons related to veterinary drug residues.
