This morning, the U.S. Food and Drug Administration (FDA) published data reporting that there were 88 total seafood entry line refusals in November (through at least November 20th) of which one (1.1%) was of shrimp for reasons related to banned antibiotics.
In addition, the FDA has also now reported an additional twenty seafood entry line refusals in October increasing the total for that month from 23 to 43. Of these additional entry line refusals, one was of shrimp for reasons related to banned antibiotics.
Beyond its reporting regarding antibiotic contaminated shrimp, the FDA indicates in its November reporting that over half of the entry lines refused last month – 45 out of 88 – were of entry lines of shrimp produced by the Indian shrimp processor Kader Exports Private Limited. All but two of the 45 entry lines were refused for the presence of salmonella (as well as either being insanitary, filthy, or lacking the manufacturer’s HACCP), with the remaining two refused for being insanitary. The FDA notes that the agency’s enforcement action began on November 18th in the Division of West Coast Imports with the refusal of 15 entry lines of shrimp from Kader Exports and that over the next two days, the Division of Southeast Imports and the Division of Northeast Imports also took action to prevent the entry of contaminated shrimp from Kader Exports entering the U.S. market. In total, the Division of West Coast Imports reported refusing 16 entry lines of shrimp from Kader Exports between November 18th and the 20th, while the Division of Southeast Imports reported refusing 18 entry lines of shrimp from Kader Exports on November 19th and 20th and the Division of Northeast Imports refused another 11 entry lines of shrimp from Kader Exports over those two days.
This is, to the Southern Shrimp Alliance’s knowledge, the first reporting by FDA indicating a joint, cooperative effort by multiple regional divisions of the agency to prevent the importation of contaminated, potentially dangerous foreign shrimp from entering the U.S. market through different ports of entry. If a coordinated action indeed took place, this would represent a substantial improvement in the FDA’s oversight of imported seafood.
As to antibiotic contamination, with two additional entry line refusals in October and November, the FDA has now reported a total of twenty-nine refusals of shrimp entry lines for reasons related to banned antibiotics through the first eleven months of 2020.
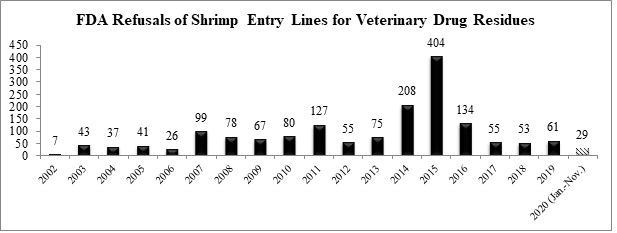
The two shrimp entry lines refused in October and November for reasons related to antibiotics were for shipments from Thailand and China:
- Phatthana Frozen Food Co., Ltd. (Thailand), a company that is currently listed on Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”) as of September 29, 2020 for enrofloxacin in its shrimp, on Import Alert 16-127 (“Detention Without Physical Examination of Crustaceans Due to Chloramphenicol”) as of October 5, 2020 for chloramphenicol in its shrimp and in its stuffed pasta products including shrimp, as well as on Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) as of October 9, 2020 for nitrofurans in its shrimp, had one entry line refused for shrimp contaminated with veterinary drug residues by the Division of West Coast Imports on October 29, 2020; and
- Fuqing Yihua Aquatic Food Co., Ltd. (China), a company that, as of September 3, 2020, was placed on the green list of Import Alert 16-131 (“Detention Without Physical Examination of Aquacultured, Shrimp, Dace, and Eel from China – Presence of New Animal Drugs and/or Unsafe Food Additives”), had one entry line refused for breaded shrimp contaminated with veterinary drug residues by the Division of West Coast Imports on November 10, 2020.
