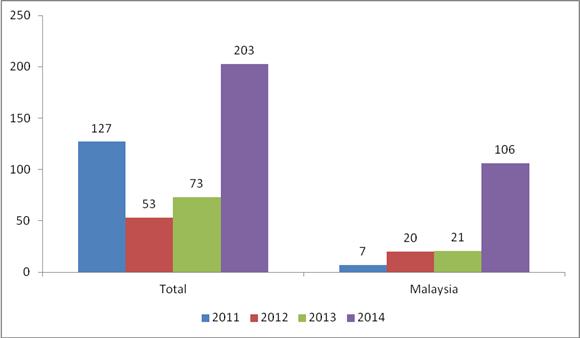
Last year, the U.S. Food and Drug Administration (“FDA”) refused 203 entry lines of imports of shrimp for reasons related to veterinary drug residues. In the three years prior (2011 through 2013), the FDA reported refusing a grand total of 253 entry lines of shrimp for the same reasons.

The substantial increase in refusals is the result of a number of factors, including the resurgence of use of veterinary drugs in shrimp aquaculture in South, Southeast, and East Asia.
The most important contributing factor, however, has been shrimp shipped from Malaysia. Although Malaysian shrimp accounted for just 3.3% of the total volume of shrimp imports in 2014 (through November, 35.1 million pounds of 1.06 billion pounds of frozen warmwater shrimp imports) and just 2.8% of the total value of shrimp imports (through November, $161 million of $5.8 billion), shrimp shipped from Malaysia comprised the majority of entry lines of shrimp refused for veterinary drug residues. With 106 entry lines of shrimp shipped from Malaysia refused in 2014, the FDA rejected more than twice the amount of entry lines of shrimp shipped from Malaysia in the prior three years (48) combined.
As should be expected, Malaysian shippers now dominate the FDA’s Import Alerts related to shrimp and veterinary drug residues. For example, Import Alert 16-129 – regarding seafood products found to contain nitrofurans – lists nineteen companies in four different countries, all for shrimp products. Fourteen of the nineteen companies listed are Malaysian. Import Alert 16-124 – regarding aquacultured seafood found to contain unapproved veterinary drugs – lists seventy-one companies from six countries for a variety of seafood products. Of these, eighteen are listed because of chloramphenicol in shrimp. The biggest contributor to that group is, again, Malaysian companies (7).
The FDA’s findings and actions with respect to shrimp shipped from Malaysia are unusual. Because of continuing abuse of banned antibiotics in shrimp aquaculture in India and Vietnam, the FDA’s findings of veterinary drug residues in shrimp shipments originating from these two countries are similarly found in the reporting of other major seafood importing markets including the European Union, Japan, Canada, Australia and Korea. In contrast, the level of rejections of shrimp shipped from Malaysia to this market is not shared elsewhere.
One likely explanation for the difference in outcomes is that Malaysia has an established history of acting as a conduit for Chinese-origin products seeking to evade import regulations in other markets. In the United States, Chinese-origin shrimp is generally subject to significant antidumping duties and Import Alert 16-131. U.S. importers and distributors seeking cheap shrimp from China may transship that merchandise through Malaysia to evade both duty payment and food safety controls. This view is supported not only by the past enforcement history of various federal agencies that have investigated transshipment, but also through the behavior of Malaysian companies once they have been placed on Import Alert by the FDA. While other foreign shrimp suppliers will go to great lengths to be removed from an Import Alert, Malaysian companies tend to simply remain on these lists. Shrimp shipped by these companies is routed through other companies with new names until they too are listed on Import Alert. In essence, the FDA’s Import Alerts have become graveyards that tell the story of Malaysian companies used to circumvent U.S. laws.
Despite this history, the FDA has only conducted limited overseas inspections of Malaysian companies purportedly processing shrimp. The agency’s inspection database indicates that the FDA has inspected only three Malaysian companies that shipped shrimp to the U.S. market since 2008 and none since 2011. More is required. And in a letter to the Commissioner of the FDA sent Friday, the Southern Shrimp Alliance asked the agency to:
- Conduct an on-the-ground assessment of Malaysia’s aquaculture industry similar to the assessment conducted of the Vietnam’s aquaculture industry in May 2012;
- Conduct significantly more on-site inspections of facilities claiming to produce shrimp in Malaysia;
- Issue a country-specific Import Alert regarding shrimp shipped from Malaysia to the U.S. market; and
- Meet with the Southern Shrimp Alliance to discuss effective strategies to eliminate the use of unapproved veterinary drugs in aquaculture.
The FDA’s considerable efforts to address banned antibiotics in shrimp led to a profound reduction in the volume of tainted shrimp sold in the U.S. marketplace. As a practical matter, the agency has demonstrated that the use of unapproved chemicals and animal-drugs in farm-raised fish products intended for human consumption, including shrimp, is amongst the FDA’s greatest concerns and, thus, a top regulatory priority. Nevertheless, the track record of Malaysian shrimp requires an even more robust response from the FDA.
Read the Southern Shrimp Alliance’s January 30, 2015 letter to the U.S. Food and Drug Administration here: https://shrimpalliance.com/wp-content/uploads/2015/02/SSA-January-30-2015-letter-to-FDA.pdf
